GRAND RAPIDS, Mich. — When it comes to winter in West Michigan, seeing the salt trucks run up and down our roads is just part of the normal cold weather routine.
However, when conditions get too cold, not even these trucks can prevent ice from forming on roads and bridges. That's because the weather can actually be too cold for salt to help keep our roads safe. Let's take a closer look at why!
The Question:
Why does salt stop working when the weather gets too cold?
The Why:
It all comes down to the chemical makeup of water and what salt is actually doing to prevent ice from forming.
To start out we need to look at water in its basic form. As a liquid the molecules in water are loosely packed and free flowing. This means it is able to run and flow freely without much resistance in itself.


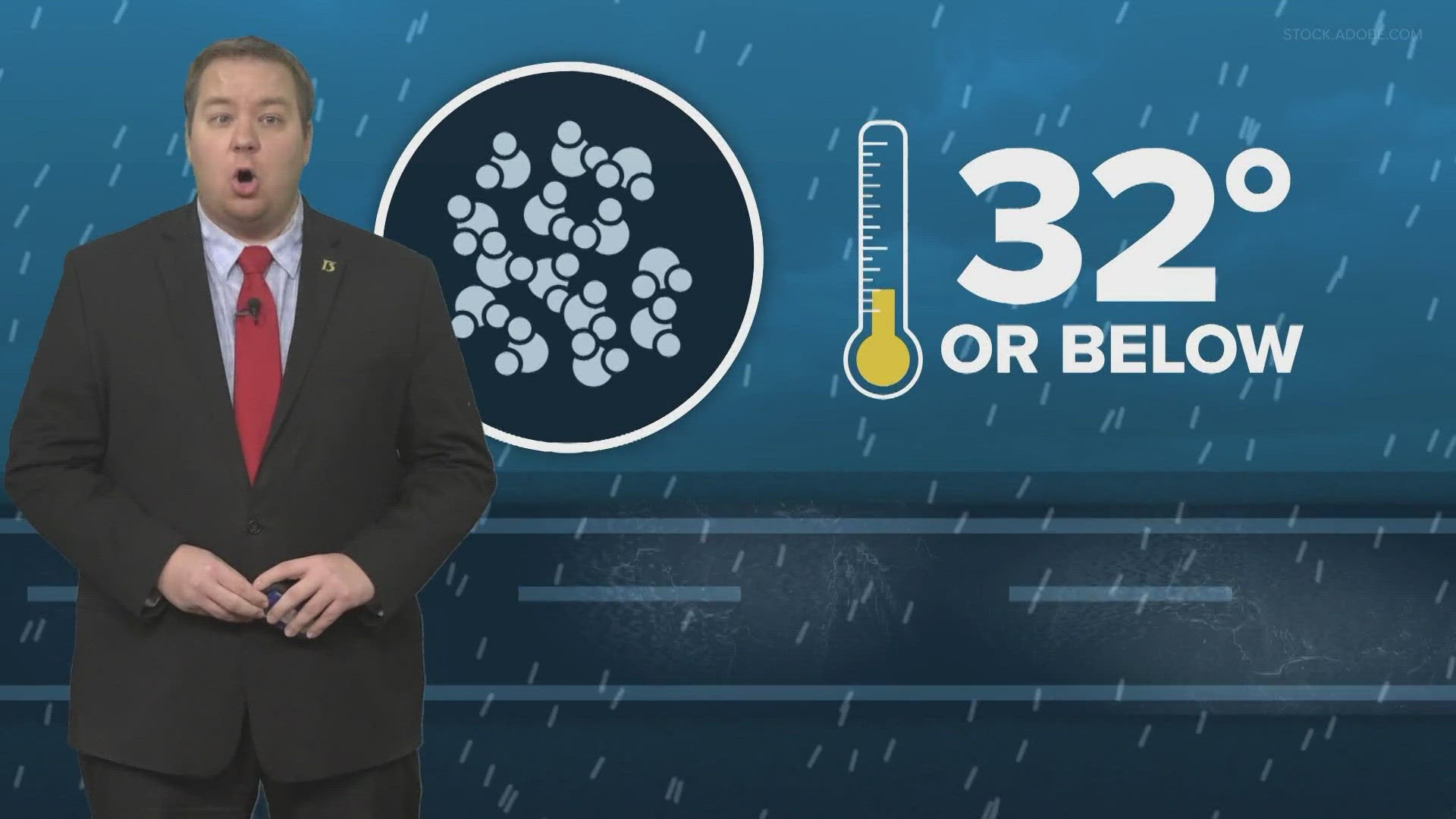
As water reaches 32 degrees or below the molecules become more tightly packed. This tightening allows ice crystals to form, and that spells bad news for drivers.
To combat this formation, we will usually add salt or a brine solution to our roads. This material is not actually melting the ice by generating any heat, but is instead causing a chemical change in the composition of the water.
As salt molecules intermix with the water molecules they prevent tight bonds from forming in the solution. This prevents ice from forming, even as the liquid temperature of the solution falls below 32 degrees.


This effect has its limits though. While some effect may be seen as temperatures plummet, most of the work that can be done from salt stops as we drop under the 15-degree mark. After this point, the amount of salt needed to melt all the ice on our roads will become impractical to actually put down.
The process will also begin to take increasingly longer and longer periods of time. This means the salt could wash or blow away before the ice actually melts.


With temperatures already having gone sub-zero this week, and not making it above 10 degrees on Monday, we have already passed the point where salt would be considered effective. As of such, we should plan on using extra caution out on what could be potentially icy and slick roads through most of this week!
-- Meteorologist Michael Behrens
Follow me on social media! Facebook Meteorologist Michael Behrens, X/Twitter @MikeBehrensWX, and Instagram/Threads @MikeBehrensWX.
Email me at: MBehrens@13OnYourSide.com
Have a 30-second video or still photo to share? We'd love to share it with everyone! Email your image to Weather@13OnYourSide.com or post it to our 13OnYourSide Facebook Page.
►Make it easy to keep up to date with more stories like this. Download the 13 ON YOUR SIDE app now.
Have a news tip? Email news@13onyourside.com, visit our Facebook page or X/Twitter. Subscribe to our YouTube channel.